
Giannakouros Thomas
Professor
Biochemistry LaboratoryAristotle University of Thessaloniki
Faculty of Sciences,
School of Chemistry
54124, Thessaloniki
Research
Our research mainly focuses on studies on post-translational modifications of proteins, especially phosphorylation.
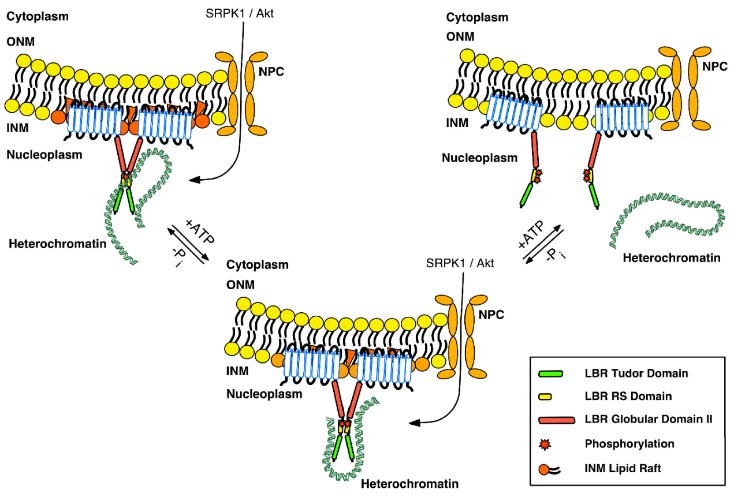
In particular, we have studied extensively the Lamin B Receptor (LBR), a protein of the inner nuclear membrane. We have shown that LBR is phosphorylated during interphase in serine residues within an Arg/Ser (RS)-rich domain by a relatively new protein kinase, SRPK1 (SR Protein Kinase 1), while during mitosis it is hyperphosphorylated by both SRPK1 and cdk1 kinase. Phosphorylation has a significant impact on the interaction of LBR with other nuclear proteins and chromatin. During spermatogenesis the combined phosphorylation of LBR and protamine 1, which also harbors an RS domain, plays a decisive role in the progressive replacement of histones by protamines. Using biochemical and bioinformatic approaches we elucidated the conformational preferences of RS dipeptides before and after their phosphorylation, thus adding to the understanding of the phosphorylation mechanism employed by SRPK1. Furthermore, we recently found that LBR is also glycosylated in serine residues adjacent to the arginine-serine dipeptides and a possible interplay between the two post-translational modifications may occur. It is clear from our data, that proteins of the inner nuclear membrane play a critical role in the attachment of chromatin to the nuclear periphery, both in somatic and sperm cells, while post-translational modifications and especially phosphorylation, regulate this interaction, thus playing a crucial role in the spatial organization of chromatin in the cell nucleus.
We also studied the phosphorylation of other substrates of SRPK1, such as the nuclear matrix protein P2P-R (Proliferation Potential Protein-Related) and ZO-2 (Zonula Occludens 2), which is a protein of the connective tissue. We identified the phosphorylation sites and studied the effect of phosphorylation in the subcellular distribution and activity of these proteins.
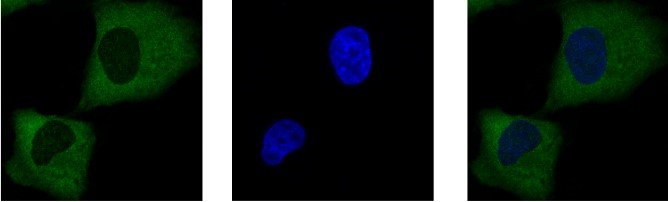
In parallel, we studied the post-translational modifications regulating the subcellular localization and activity of SRPK1. This kinase appears to have a complex regulation mechanism that involves phosphorylation, acetylation, and the development of disulfide bonds in the spacer domain that separates the two catalytic subunits of the kinase. SRPK1 has been recently found to be overexpressed in multiple cancers, while in terms of molecular mechanism, SRPK1 seems to act heterogeneously, and has been reported to affect several processes in different cancers. Given this pleiotropic mode of action, the main goal of our current research, in collaboration with Eleni Nikolakaki’s team, is the elucidation of the SRPK1 signaling pathways in correlation with the post-translational modifications of the kinase and its substrates.

